
When a child takes a medicine, their body doesn’t just shrink down to fit an adult’s response. Kids aren’t small adults - and that simple truth changes everything about how drugs work in their bodies. A cough syrup that calms an adult might make a toddler hyper. An antibiotic that causes mild stomach upset in you could send a baby to the hospital. Every year, pediatric medication side effects land nearly 10% of hospitalized children, and nearly half of those cases are life-threatening. This isn’t rare. It’s predictable - if you know how children’s bodies process drugs differently.
Why Children’s Bodies Handle Drugs So Differently
From the moment a baby is born, their body is changing at a speed no adult ever experiences. Their liver, kidneys, and brain are still building their machinery. That means drugs don’t just sit there and do their job - they get absorbed, broken down, and flushed out in ways that shift dramatically with age. A newborn has only 30-40% of the adult liver enzyme activity needed to break down medications. By six months, some of those enzymes are working at 200% of adult levels. That’s why a dose that’s safe for a 5-year-old can be deadly for a 6-month-old. The same drug, same milligram amount, but completely different results. Body composition matters too. Babies are made of more water - up to 80% compared to 60% in adults. That means water-soluble drugs spread wider in their bodies, often requiring lower doses per kilogram. Fat-soluble drugs? They behave differently because babies have less fat. And their blood-brain barrier? Still developing. That’s why sedatives and antihistamines can cause extreme drowsiness or even seizures in young kids when they’d just make an adult a little sleepy.The Most Dangerous Drugs for Children
Not all medications are created equal when it comes to kids. Some have been used for decades without proper pediatric testing. The Key Potentially Inappropriate Drugs in Pediatrics - known as the KIDs List - was created by Mayo Clinic researchers to flag the worst offenders. These aren’t just side effects. These are preventable emergencies.- Loperamide (Imodium): Used for diarrhea, but in kids under 6, it can slow the heart to a stop. Over 100 cases of fatal cardiac events have been reported to the FDA.
- Aspirin: Even a single dose in a child with a viral infection like the flu or chickenpox can trigger Reye’s syndrome - a rare but often fatal liver and brain disorder.
- Codeine: It’s metabolized by a liver enzyme called CYP2D6. Some kids are ultra-rapid metabolizers - meaning they turn codeine into morphine faster than their body can handle. One in 30 children falls into this high-risk group. Respiratory arrest can happen within hours.
- Benzocaine teething gels: These numbing gels can cause methemoglobinemia - a condition where blood can’t carry oxygen. Between 2006 and 2011, over 400 cases were reported to the FDA, mostly in children under 2.
- Montelukast (Singulair): Used for asthma, but the Columbia University study found a 3.2-fold increase in psychiatric side effects - nightmares, agitation, depression - during the second year of life.
Why So Many Drugs Are Used Off-Label in Kids
Only about half of all medications prescribed to children have been formally studied in pediatric populations. That means doctors are guessing - using adult dosing formulas, adjusting by weight, hoping it works. The American Academy of Pediatrics estimates that 50-75% of drugs used in children are off-label. Why? Because drug companies have little financial incentive to test medicines on kids. Clinical trials take longer. Parents are harder to recruit. Regulatory hurdles are high. Even though the FDA and NIH have pushed for pediatric studies since the 1990s - with laws like the Best Pharmaceuticals for Children Act - progress is slow. Between 2002 and 2022, only half of drugs approved for adults got updated labels for children. The result? Neonatal intensive care units (NICUs) are filled with babies on drugs never tested for them. A 2020 study found 79% of medications used in NICUs are off-label. For children with rare diseases, the number is worse: 95% have no FDA-approved treatment at all.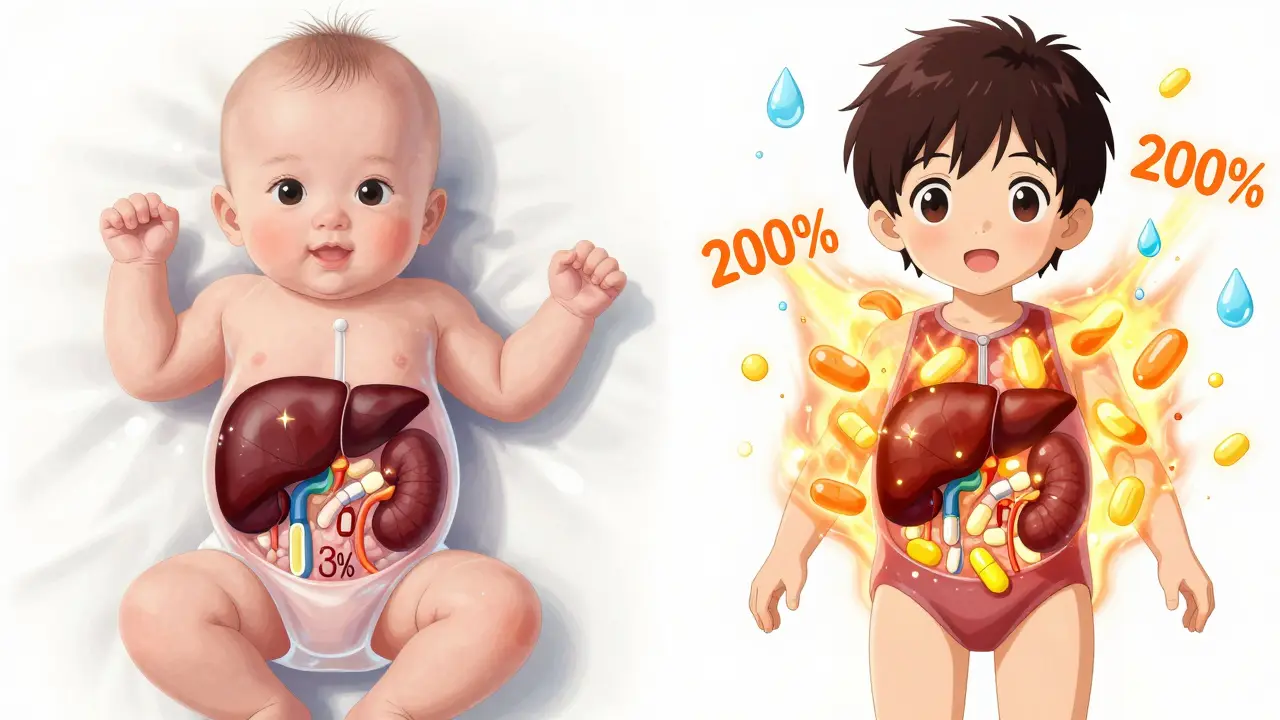
When Side Effects Are Normal - and When They’re Not
Not every reaction means danger. Many kids get mild side effects when starting a new medicine: nausea, drowsiness, a rash, or a little diarrhea. These often fade within a few days as the body adjusts. That’s normal. But knowing when to act is critical. Here’s what needs immediate attention:- Difficulty breathing - even if it’s slight. Could be an allergic reaction.
- Swelling of the face, lips, or tongue - a sign of anaphylaxis.
- Rapid or irregular heartbeat - especially if the drug isn’t supposed to affect the heart (like antibiotics).
- Extreme drowsiness or unresponsiveness - beyond normal sleepiness.
- Yellowing of skin or eyes - possible liver damage.
- Seizures or unusual movements - especially after starting a new psychiatric or GI drug.
Who’s Most at Risk?
Some kids are more vulnerable than others. Research shows three big risk factors:- Young age - especially under 2. Their organs are still building.
- Chronic conditions - kids with asthma, epilepsy, or heart disease often take multiple drugs, increasing the chance of dangerous interactions.
- Polypharmacy - taking three or more medications at once. The more drugs, the higher the risk of unexpected reactions.

What Parents and Doctors Can Do
You can’t control everything - but you can control a few key things.- Ask: “Has this been tested in children?” If the answer is no, ask if there’s a safer alternative.
- Use weight-based dosing - never guess. A 10-pound infant needs a different dose than a 40-pound toddler. Always confirm the milligrams per kilogram.
- Keep a medication diary - write down what was given, when, and any symptoms that follow. This helps doctors spot patterns.
- Use the KIDs List - it’s free and public. Search it before accepting a new prescription.
- Report side effects - if your child has a bad reaction, file a report with the FDA’s MedWatch program. These reports help build the database for future safety.
The Future: Better, Safer Medicines for Kids
Change is coming - slowly, but it’s coming. The Columbia University team launched KidSIDES in 2023 - a free database that maps drug side effects to specific childhood age groups. It shows you not just that a drug is risky, but when it’s most dangerous. A drug might be safe for a 4-year-old but deadly for a 1-year-old. That’s the kind of detail that saves lives. The FDA’s 2023 Pediatric Action Plan is pushing for more use of computer modeling to predict how drugs behave in kids, reducing the need for risky trials. And the NIH is funding a $15 million project to create age-specific pharmacogenomic guidelines - meaning someday, a child’s DNA might tell doctors exactly which drug and dose is safest for them. But until then, the burden falls on parents and doctors. We need to stop treating kids like mini-adults. We need to demand better. We need to ask harder questions. Because every child deserves a medicine that’s been studied for them - not just guessed at.What to Do If Your Child Has a Reaction
- Stop the medication immediately if you see signs of a severe reaction (breathing trouble, swelling, seizures).
- Call your pediatrician or go to the ER - don’t wait to see if it gets better.
- Write down everything: the drug name, dose, time taken, symptoms, and when they started.
- Take the medicine bottle with you to the doctor.
- File a report with the FDA’s MedWatch program. Your report helps protect other children.
Why are children more sensitive to drug side effects than adults?
Children’s bodies are still growing. Their liver and kidneys don’t process drugs the same way as adults, their brain barrier is still developing, and their body composition (more water, less fat) changes how drugs spread. Even small differences in enzyme activity can turn a safe adult dose into a dangerous one for a child.
Is it safe to give my child a drug that’s approved for adults?
Not without checking. Many adult drugs haven’t been tested in children. Even if you adjust the dose by weight, the way the drug behaves in a child’s body may be completely different. Always ask your doctor if the drug has pediatric labeling or if it’s being used off-label.
What should I do if my child gets a rash after taking medicine?
Mild rashes that appear within the first few days and don’t spread or itch badly may be temporary. But if the rash is raised, itchy, spreads quickly, or is accompanied by swelling or breathing trouble, stop the medicine and seek medical help immediately. It could be an allergic reaction.
Can I reduce the dose if I’m worried about side effects?
Never change the dose on your own. Under-dosing can make the drug ineffective. Over-dosing can be dangerous. Always talk to your doctor first. If side effects are a concern, they may switch the drug, adjust the schedule, or monitor your child more closely.
What is the KIDs List, and how can I use it?
The KIDs List is a free, science-backed list of medications with higher risks for children, created by Mayo Clinic researchers. You can search it online to check if a prescribed drug is flagged. It helps you ask better questions before giving your child a new medicine.
Are there any new tools to help parents avoid dangerous drug reactions?
Yes. The Pediatric Drug Safety Portal (PDSportal) and KidSIDES database, both launched in 2023, let healthcare providers and parents see which drugs carry risks at specific ages. These tools show not just that a drug is risky, but when - like how montelukast poses the highest psychiatric risk during the second year of life.

Winni Victor
December 24, 2025 AT 22:50So let me get this straight - we’re giving toddlers drugs that could stop their hearts… and the FDA’s like ‘eh, we’ll get around to testing it someday’? I mean, I get that Big Pharma doesn’t wanna spend money on kids, but come ON. My cousin’s kid nearly died on loperamide because the pharmacist didn’t even blink when the mom said ‘just give him half a pill.’ Half a pill. For a 2-year-old. That’s not negligence - that’s a death sentence with a prescription pad.
Linda B.
December 25, 2025 AT 23:51The real story here is that pediatric drug trials are a government-backed cover for corporate control. The KIDs List? A distraction. The real culprits are the WHO, the CDC, and the pharmaceutical lobbying groups who’ve been quietly replacing childhood immunity with chemical dependency since the 80s. They don’t want you to know that most of these drugs were originally developed for military use. That’s why they’re so potent. That’s why they’re so dangerous. That’s why they’re still on the shelf.
Christopher King
December 26, 2025 AT 13:33Let me ask you something. If a child’s body is a completely different operating system than an adult’s - why are we still running adult software on it? We wouldn’t plug a Windows 11 app into a DOS machine and expect it not to crash. But we do this every single day with kids and meds. It’s not just negligence - it’s a systemic failure of imagination. We’re not just under-dosing or over-dosing - we’re thinking in the wrong language entirely. The answer isn’t more studies. The answer is a complete rewrite of pediatric pharmacology. And no one’s got the guts to do it.
Katherine Blumhardt
December 26, 2025 AT 21:30my kid got a rash after singulair and i just thought it was allergies until i read this and i was like oh my god i almost killed him. i deleted the app and called the dr and they were like ‘oh yeah we know it happens’ and i was like YOU KNOW AND YOU STILL PRESCRIBE IT?? i hate medicine. i hate doctors. i hate this system. i just want my kid to be safe. why is this so hard?
sagar patel
December 27, 2025 AT 06:57Children metabolize drugs differently due to immature hepatic and renal systems. This is well documented in pharmacokinetic literature since the 1970s. The real issue is not lack of knowledge but lack of enforcement. Regulatory agencies prioritize market access over safety. Off-label prescribing is not an accident - it is institutionalized risk transfer from manufacturers to parents.
Harbans Singh
December 27, 2025 AT 12:20I’ve been a nurse for 18 years and I’ve seen this over and over. A baby on codeine after tonsil surgery - gone in 12 hours. A toddler on benzocaine gel - stopped breathing. We know this. We’ve always known this. But the system doesn’t care until a child dies. And even then, it’s just another footnote. I wish more parents knew about the KIDs List. I give it out like candy now. It’s the only thing keeping me sane.
Rick Kimberly
December 29, 2025 AT 06:33The data presented is both compelling and alarming. It is empirically evident that pediatric pharmacokinetics and pharmacodynamics diverge significantly from adult norms, necessitating age-stratified dosing protocols and rigorous clinical validation. The absence of FDA-approved labeling for the majority of pediatric prescriptions constitutes a critical gap in evidence-based practice. Further, the underrepresentation of pediatric populations in clinical trials remains a persistent ethical and methodological challenge. The implementation of pharmacogenomic screening may offer a path forward, but only if accompanied by systemic reform in regulatory incentives.
Terry Free
December 30, 2025 AT 18:59You people are freaking out over a pill. Kids get sick. Pills fix it. If your kid has a bad reaction, maybe you’re the problem. I gave my daughter aspirin when she had the flu and she lived. She’s 22 now. You think you’re so smart because you read a blog? Go back to your yoga classes and stop scaring parents. The system works fine. You just don’t like being told what to do.
Lindsay Hensel
January 1, 2026 AT 10:11This is heartbreaking. And necessary. Thank you for writing this.
Every child deserves to be seen as a child - not a mini-adult.
We must do better.
Not because it’s convenient.
But because it’s right.
Sophie Stallkind
January 1, 2026 AT 18:29While the emotional weight of this post is undeniable, the underlying argument hinges on a fundamental assumption: that off-label prescribing is inherently dangerous. This is not necessarily the case. Many off-label uses are supported by clinical consensus and expert guidelines. The issue is not the off-label status per se, but the absence of standardized, evidence-based pediatric dosing protocols. The solution lies not in fear, but in structured, collaborative research between clinicians, regulators, and pharmacologists.
Bailey Adkison
January 3, 2026 AT 05:42Let’s be real - this whole thing is a scam. The KIDs List? Made by Mayo Clinic. Who funds Mayo? Big Pharma. The ‘dangerous drugs’ they list? The ones that aren’t patented anymore. The real villains? The new expensive biologics they push instead. You think they care about your kid? They care about your insurance card. They want you to trade a $2 generic for a $2000 monthly infusion. This isn’t about safety. It’s about profit.
Michael Dillon
January 3, 2026 AT 17:37My son took montelukast for a year. Nightmares every night. Screaming at 3 a.m. like he was being chased by demons. We stopped it. He slept like a baby. No one told us. No one warned us. I’m not mad. I’m just… tired. Why is it always the parents who have to figure this out? Why isn’t there a simple app? A warning label? A goddamn sign? We’re not doctors. We’re just trying to keep our kids alive.
Gary Hartung
January 4, 2026 AT 05:22It is, without a shadow of a doubt, an absolute travesty - a catastrophic failure of medical ethics, pharmacological foresight, and institutional accountability - that we continue to administer unvalidated, untested, and potentially lethal pharmaceutical agents to our most vulnerable demographic: pediatric patients. The fact that this is even a conversation - that we are still debating whether to treat children as biological entities with unique physiological parameters - speaks volumes about the profound intellectual and moral decay of modern medicine. This is not a gap. This is a chasm. And we are all falling into it - willingly, complacently, and with the hollow comfort of a well-rehearsed, corporate-approved talking point.